by Mansur Zhussupbekov
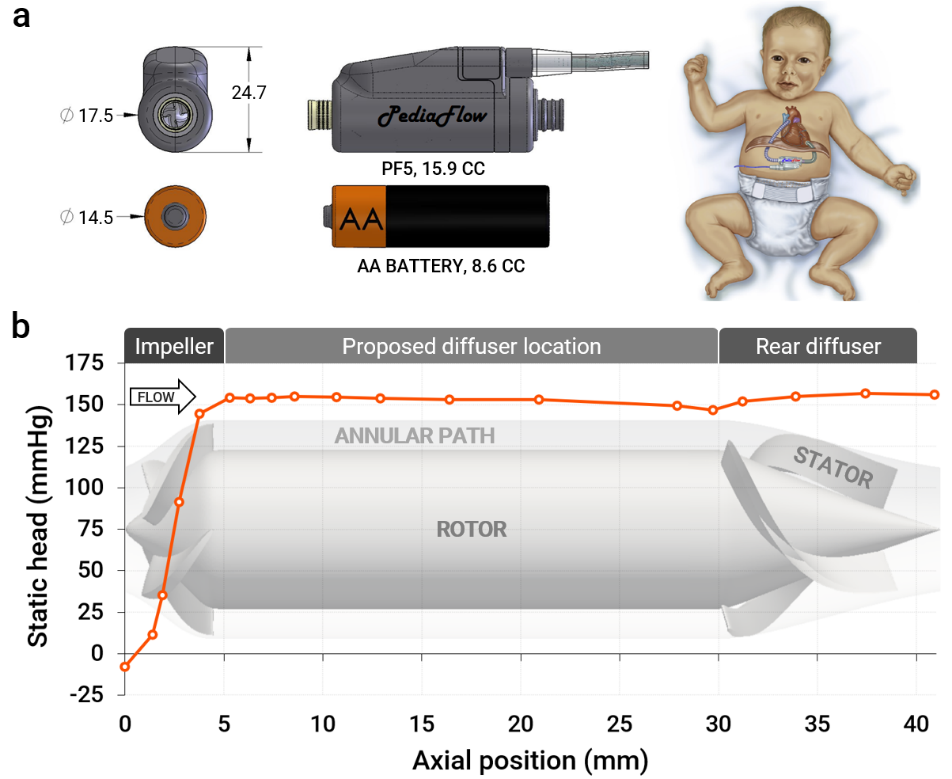
PediaFlow is an implantable heart pump, also known as ventricular assist device (VAD), intended to provide hemodynamics support for infants and young children with heart failure. The fifth-generation PediaFlow (PF5) is a miniature, magnetically levitated pump approximately the size of an AA battery, capable of delivering flows ranging from 0.5 to 4.0 L/min. Developing miniaturized blood pumps like the PediaFlow requires navigating a delicate balance of hydraulic performance, biocompatibility, and manufacturability. This problem lends itself well to automated shape optimization, hence why in our latest work, we turned to CFD-driven automated shape optimization using CAESES to tackle a key challenge – improving pressure recovery in the pump’s flow path while maintaining excellent biocompatibility.
A key feature of the PediaFlow is its fully magnetically levitated rotor, which eliminates mechanical bearings and significantly enhances biocompatibility. However, achieving sufficient magnetic stiffness requires an extended rotor length, resulting in an unconventional design with a long axial gap between the impeller and diffuser stages. This extended path causes energy losses as blood traverses from impeller to diffuser, further exacerbated by Taylor vortices that develop within the annular gap. To address these losses, we proposed adding a set of stationary diffuser blades (front diffuser) immediately downstream of the impeller to convert dynamic head into static pressure more efficiently. Our hypothesis was that this would allow the pump to achieve its desired operating point at a lower rotor speed.
When optimizing blood pumps, any gains in hydraulic performance must be carefully weighed against potential impacts on hemo-compatibility (the pump’s compatibility with blood). Of particular concern is hemolysis – the damage to red blood cells caused by exposure to supra-physiologic levels of shear stress. This inherent trade-off between performance and blood damage makes this task particularly well-suited for multi-objective optimization.
Diffuser Parameterization and Optimization Worflow
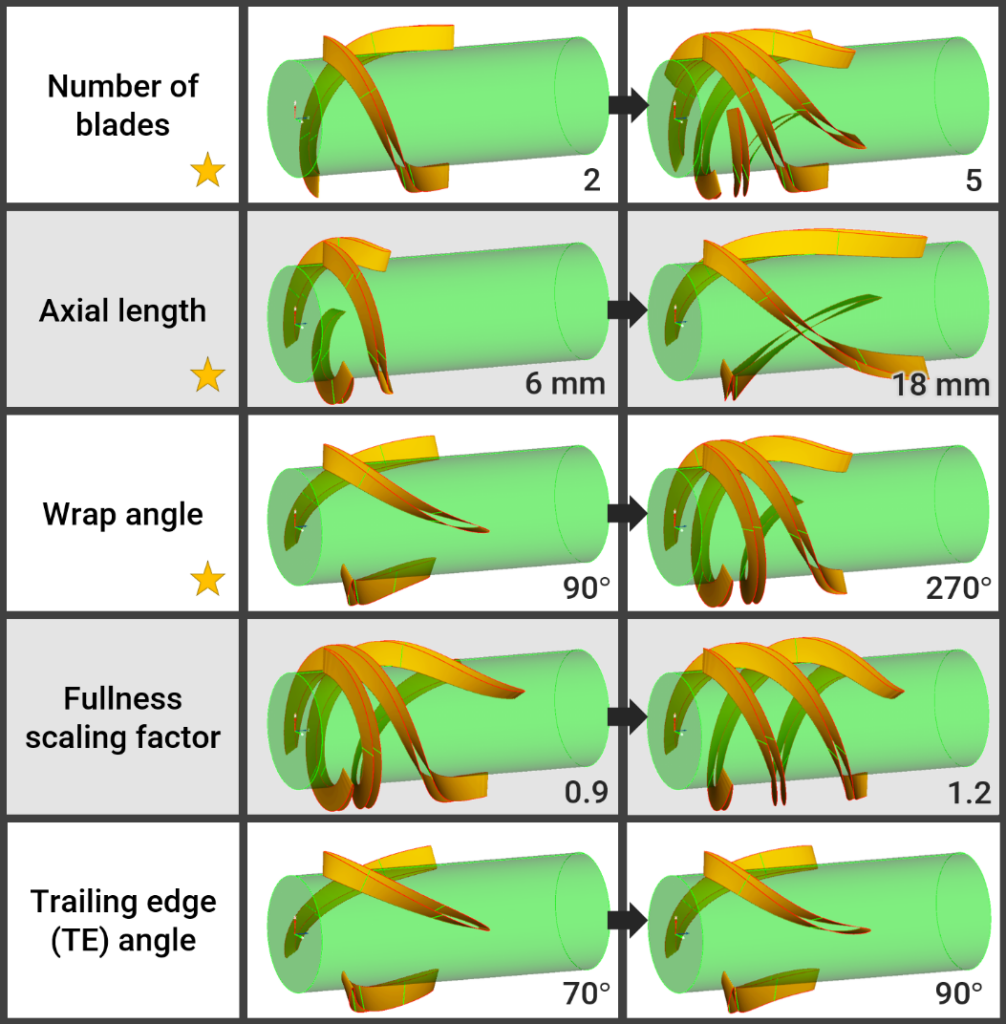
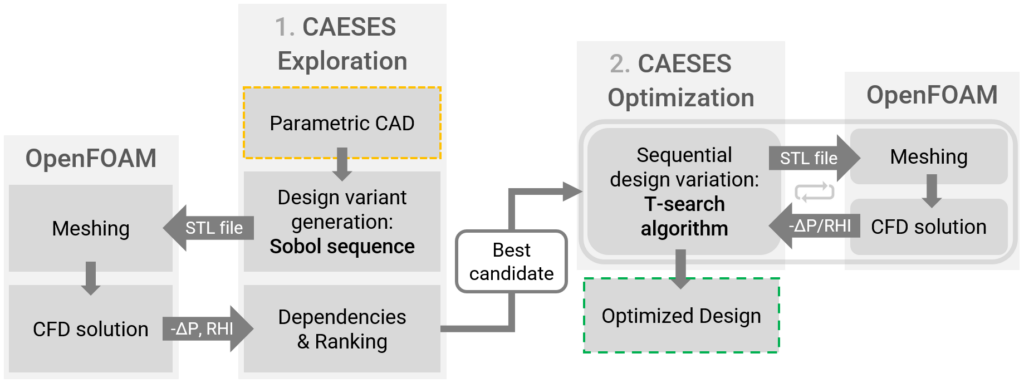
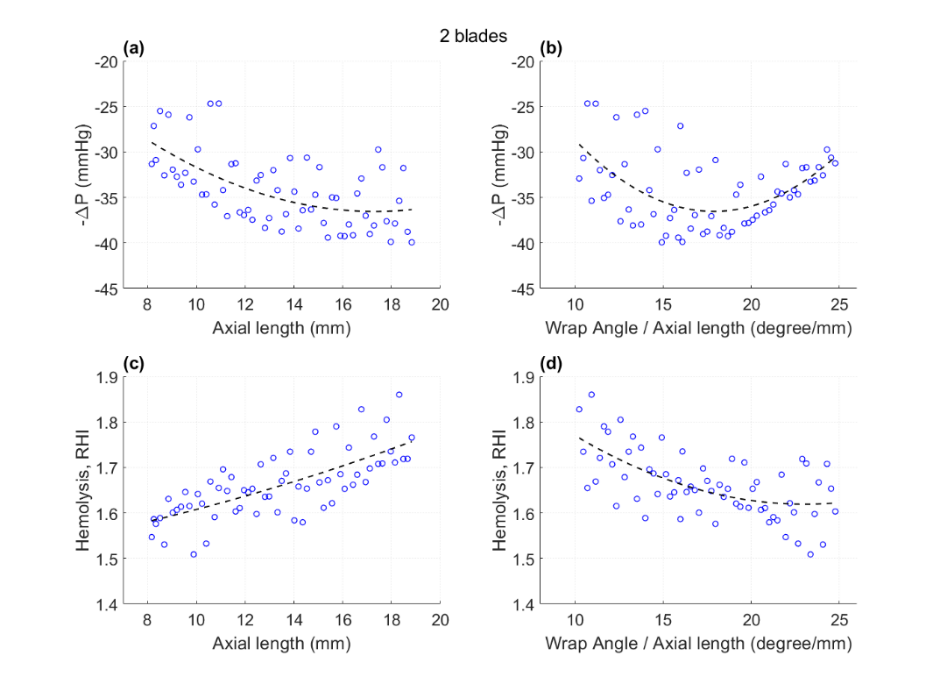
Using CAESES, we were able to fully parameterize the geometry of these new diffuser blades, see the effects of the selected parameters and their ranges in the picture above. The CAESES module was coupled with OpenFOAM for meshing and CFD and controlled the optimization process. This allowed us to systematically explore the design space and identify the optimal diffuser configuration that maximized pressure recovery while minimizing hemolysis.
Results and Findings
Our key findings from this effort include:
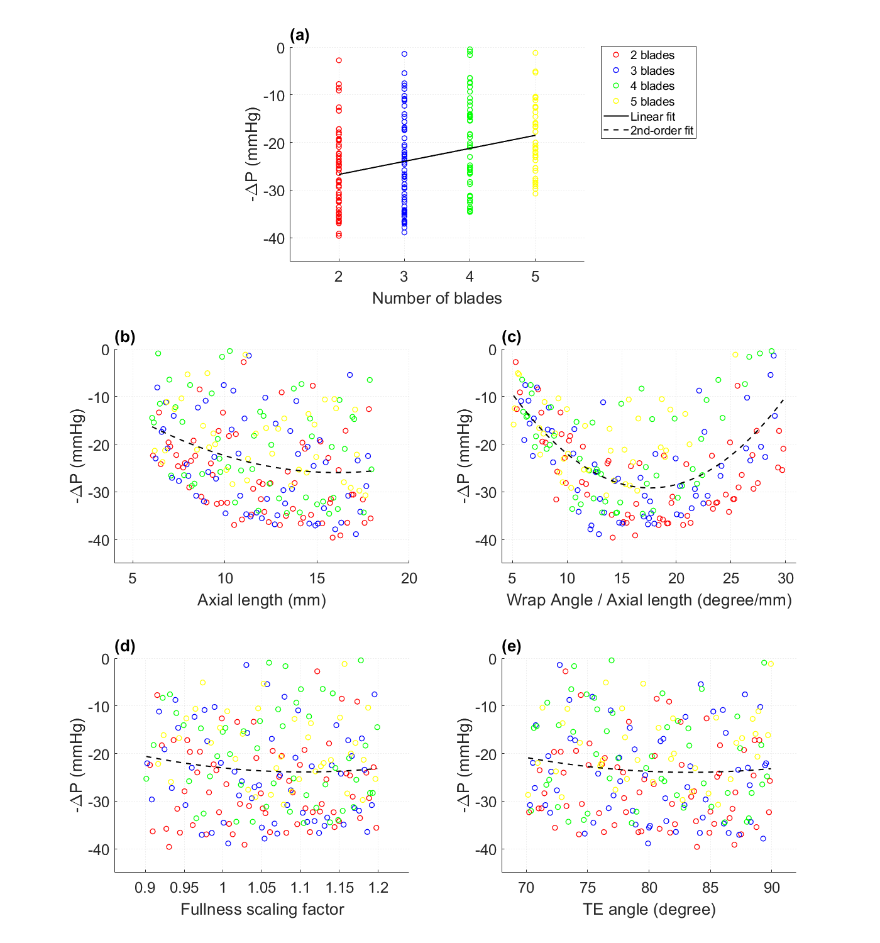
- Diffuser designs with fewer blades (2-3) outperformed those with more blades (4-5) in terms of pressure recovery.
- Increasing the blade axial length generally improved pressure rise but also increased hemolysis (expressed as RHI – relative hemolysis index).
- There was an optimal range for the wrap angle to axial length ratio that balanced pressure recovery and blood damage.
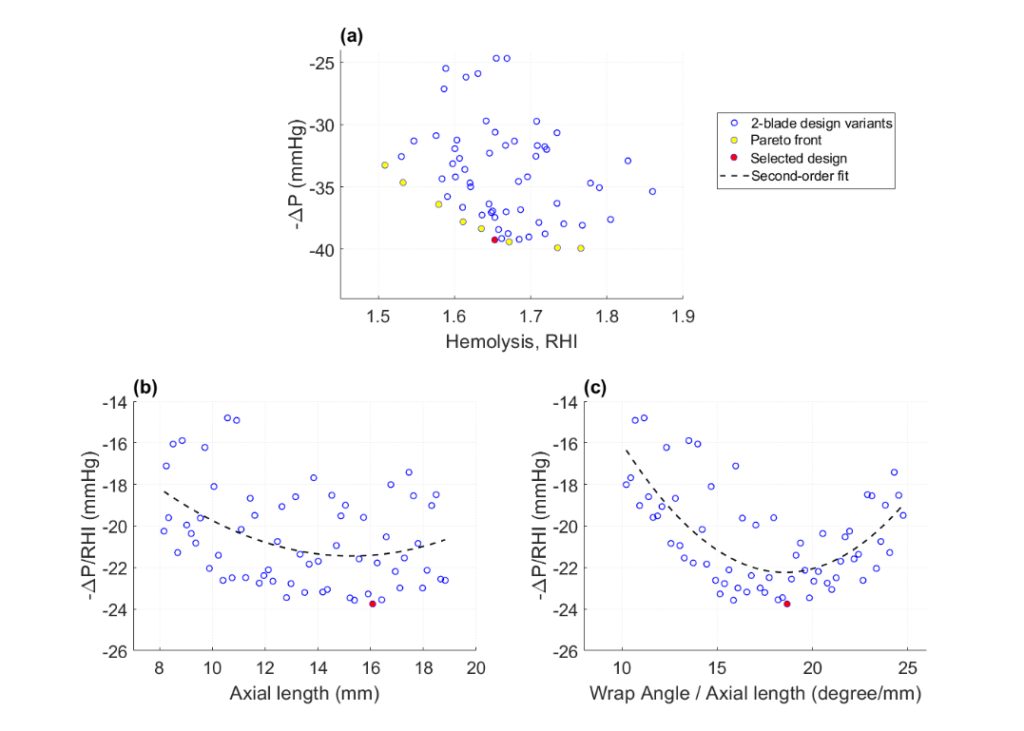
- Plotting pressure recovery vs hemolysis revealed a set of Pareto-optimal solutions that balanced hydraulic performance and hemo-compatibility. Using the utility function that combined the two objectives into the ratio of -∆P to RHI allowed selecting the best design candidate.
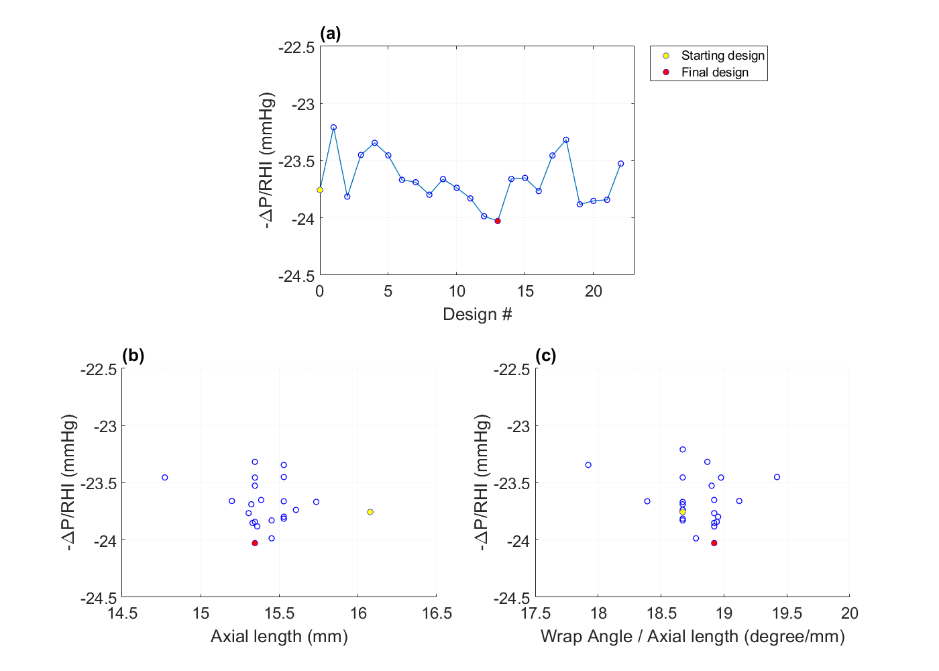
- The best candidate from the Exploration stage was further optimized using the T-search algorithm. The T-search optimization improved the ratio -∆P/RHI from 23.76 to 24.0, primarily attributed to the reduction in hemolysis from RHI 1.65 to 1.63.
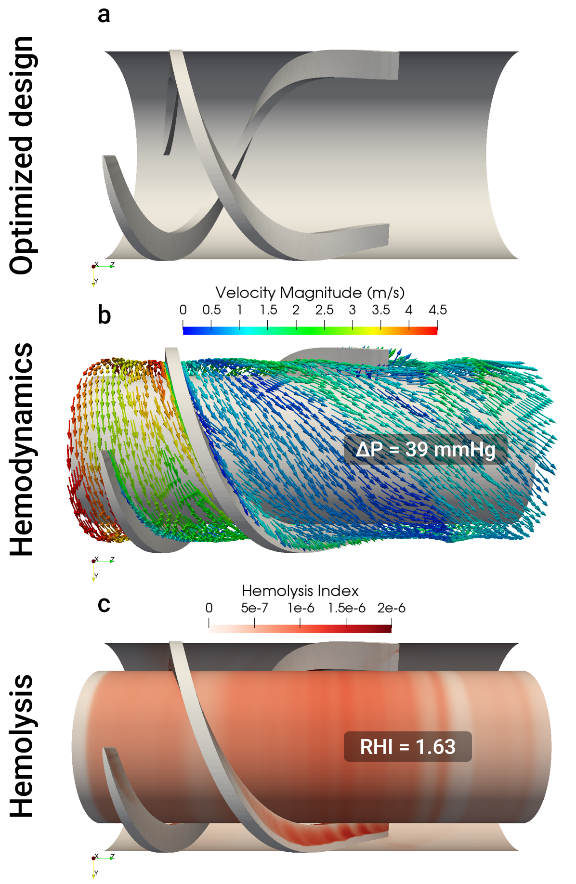
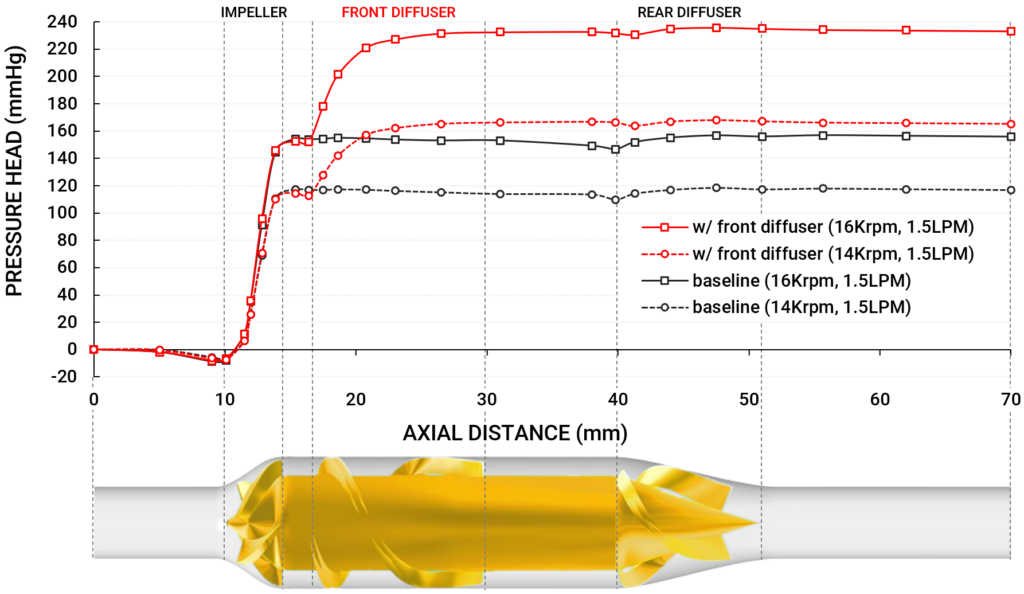
By combining these insights, we converged on an optimized 2-blade diffuser design that produced a 39 mmHg pressure rise in an isolated stage – a 26% increase from the baseline pressure head at 14,000 RPM. Importantly, we were also able to assess the hemolytic performance of this design and identify the optimal compromise between pressure recovery and blood damage.
To verify the performance of the optimized front diffuser in the full pump, we incorporated it into a CFD simulation of the complete PF5 flow path. The results were remarkable: the pump with the added front diffuser stage achieved the target OP of 1.5 L/min at 160 mmHg while running at just 14,000 RPM, compared to the 16,000 RPM required by the baseline design. This reduction in operating speed, combined with improved flow guidance, yielded two significant benefits: the hydraulic efficiency increased from 26.3% to 32.5%, and predicted hemolysis decreased by 31%. These results suggest that the mid-stator not only enhances the pump’s performance but also improves its hemo-compatibility.
Conclusion and Outlook
CAESES’s robust optimization framework, coupled with OpenFOAM, proved instrumental in this work. The ability to rapidly iterate on the blade geometry and seamlessly connect to high-fidelity CFD simulations allowed us to navigate the complex design space efficiently. Looking ahead, we plan to expand our optimization to consider off-design operating conditions and assess the impact on overall pump efficiency and hemo-compatibility. As we continue refining and validating this solution, we hope it will contribute to our broader efforts to deliver safer and more effective circulatory support devices for pediatric patients.
About the Author
Mansur Zhussupbekov is a Biomedical Engineer with a Ph.D. degree from Cornell University, specializing in implantable cardiovascular devices and with comprehensive experience in design and testing of early-stage Class III devices for treatment of heart failure. He is currently working as an R&D Postdoctoral Associate for the PediaFlow Consortium at the Meinig School of Biomedical Engineering at Cornell University.
Notable contributors to this project are: Jingchun Wu, PhD (Advanced Design Optimization, LLC, Irvine, CA, USA), Greg W Burgreen, PhD (Center for Advanced Vehicular Systems, Mississippi State University, Starkville, MS), Jeongho Kim, PhD (Department of Biomedical Engineering, Daejeon Institute of Science and Technology, Daejeon, Korea), led by James F Antaki, PhD (Meinig School of Biomedical Engineering, Cornell University, Ithaca, NY, USA).
Learn More
See this overview for the possibilities and capabilities that CAESES offers for medical applications.